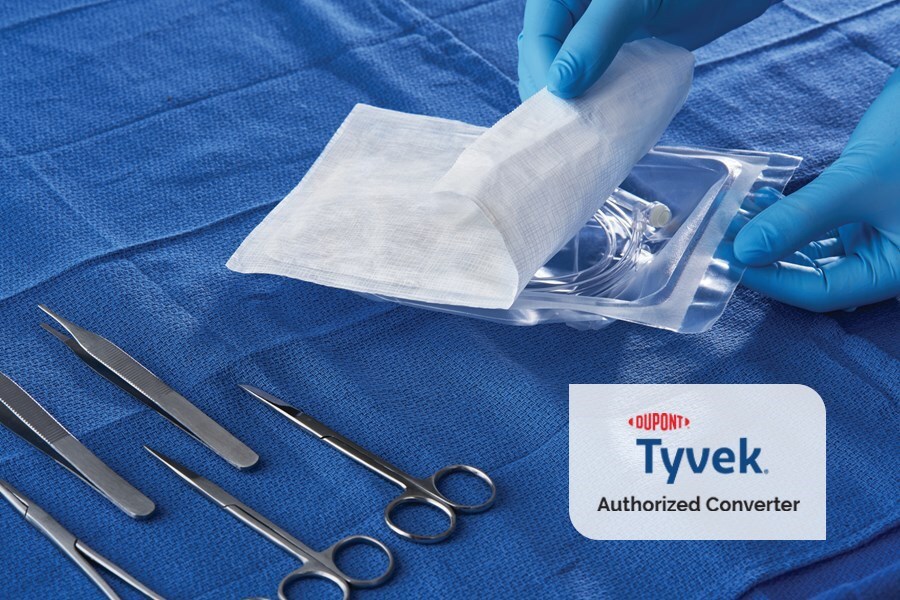
Sterile Packaging
DuPont™ Spectrum™ provides pouches, header bags, and lids made with DuPont™ Tyvek®, providing the highest level of protection. We are one of the few authorized DuPont™ Tyvek® converters in the world for the healthcare industry.
Medical device companies are turning to trusted supply chain partners to determine the best approach to sterilize and package their products.
Spectrum™ is a custom, scalable manufacturer of flexible packaging. One of the most experienced companies in the industry, Spectrum™ launched its blown film, extrusion, and conversion operations 70 years ago.
Spectrum™ offers mono-layer and co-extruded films and film conversion in a variety of polyolefin resins for a wide array of sterile bags, pouches, and lids. Our sterile barrier packaging is available in peelable, tear-open, breathable, and non-breathable pouches.
Most of our packaging is made with Tyvek®, which provides superior tear strength and puncture resistance. Our strong, long-term relationship with DuPont™ is reflected by the fact that we are one of the few DuPont™ Tyvek® Converters in the world for the healthcare industry.
Tyvek® can withstand challenging sterilization and distribution environments. Tyvek® packages are ideal for protecting sensitive medical devices and can be manufactured to accommodate irregularly shaped products. Styles 1073B, 1059B, and 2FS are made of HDPE, which can be recycled and help manufacturers meet their sustainable design goals to reduce their environmental footprint.
For advice from our packaging experts regarding best practices for graphic design and printing of medical packaging, including the electronic submittal of final artwork, please consult Guidelines for Medical Packaging Graphic Design.
Customers have rated Spectrum™ as an “exceptional” organization for meeting their complete product needs and providing outstanding service, quality, and delivery. Because our product return rate is less than 0.003% and we are a certified dock-to-stock vendor, many customers have eliminated incoming inspections of our products, knowing they will be of the highest quality and ready to use.
Our sterile medical packaging/products, including pouches, header bags, and lids, as well as specialty film conversions for these products, are made from Tyvek®, paper, foils, and polyethylene.
Pouches
Peel pouches are made in all medical styles, including 1073B, 1059B, and 2FS™.
Header Bags
Ideal for heavy and bulky medical devices, header bags feature strong linear low-density polyethylene with uncoated and coated headers made with Tyvek®.
Die-Cut/Square-Cut Lids
Die-cut and square-cut lids are made with Tyvek® and feature coated CT1073, CT1059, and CT2FS. For maximum device protection, consider a thermoformed tray with a die-cut or square-cut lid, which protects your device and still permits ease of sterilization.
A form-fill-seal (FFS) machine is an effective packaging process that creates the package, fills it with the product, and then seals it—all in one easy operation. Flexible film is the preferred packaging material, which is typically heat-sealed. Ultrasonic inspection technology can be used to identify incredibly small defects or contaminants in the seal.
Popular materials for FSS packaging are LLDPE, LLPE, and Surlyn. This type of packaging is also a good choice for sensitive or delicate medical products for the infusion, blood management, orthopedic, and medical-surgical markets.
Understanding the chemical interactions between the selected packaging material and the selected sterilization method is critical for success. The material must be able to withstand repeated cycles in the harsh sterilization environment. For gas-based sterilization methods, the packaging material must provide enough breathability to ensure sterilization is complete, as well as any adjustments for pressure variations that might occur during the process.
Spectrum™ engineers work closely with customer development teams to create specialized packaging solutions for their products, especially those that have sensitive components. Key factors to consider for customization include:
The ability to customize through the utilization of different materials is also a key advantage when dealing with supply chain shortages. Spectrum™ works with a high-quality network of reliable material providers to find effective alternative materials that will still deliver or exceed desired performance expectations.
As their products get smaller and more complex, medical device manufacturers reach out to their contract manufacturers to collaborate on design, production, and packaging. They respect their CM’s knowledge and expertise, which spans a wider range of applications across multiple industries.
Packaging design and validation studies can take months to complete, so it is beneficial and cost-effective to include CMs up-front in the design phase.
Drawing on combined knowledge and expertise about material behavior and process and packaging methods is the best way to develop a packaging system that fully protects a single product or a class of products during its shipping journey.
Spectrum™ works closely with MDMs as a trusted technology and material advisor to develop and manufacture their most innovative and complex products. Our highly experienced engineering teams are eager to share their knowledge, experience, and problem solving skills to deliver solutions that meet or exceed a product’s requirements for packaging performance, functionality, and product protection.
Contact us to learn how we can help you with your next flexible film and packaging project.
Sterile packaging shelf life varies based on material selection, sterilization method, and storage conditions. Materials like DuPont™ Tyvek® provide extended barrier protection when properly stored. Specific shelf life determination requires validation studies that consider environmental factors, handling requirements, and device characteristics, with regular testing ensuring maintained sterility throughout the validated period.
Medical device sterile packaging includes peelable pouches, breathable and non-breathable configurations, header bags for bulky items, and die-cut lids for thermoformed trays. Form-fill-seal (FFS) systems utilizing flexible films provide additional solutions for specific applications. Each format offers distinct advantages for different medical devices, ensuring sterile barrier integrity while accommodating various shapes and protection requirements.
Sterile packaging maintains a validated barrier after terminal sterilization, protecting devices through distribution and storage. In contrast, aseptic packaging involves assembling sterilized components in a controlled environment, typically for products unsuitable for terminal sterilization. The primary distinction lies in sterilization timing and specific barrier requirements for each method.
Medical device storage duration is established through stability studies evaluating packaging integrity and device functionality. Validated storage periods consider packaging material stability, sterilization effects, and controlled environmental conditions. Manufacturers must conduct real-time aging studies and maintain proper storage protocols to ensure devices retain their performance throughout the designated shelf life.
We’re happy to help with your projects in any way we can. Contact us and we’ll email you back the information you’re looking for, or we’ll schedule a call to discuss with you in more detail.