


Catheter Technologies
We offer a full range of vertically integrated catheter technologies and secondary services. Whether you need a catheter that’s braided or coiled, steerable or deflectable, we can meet your needs.
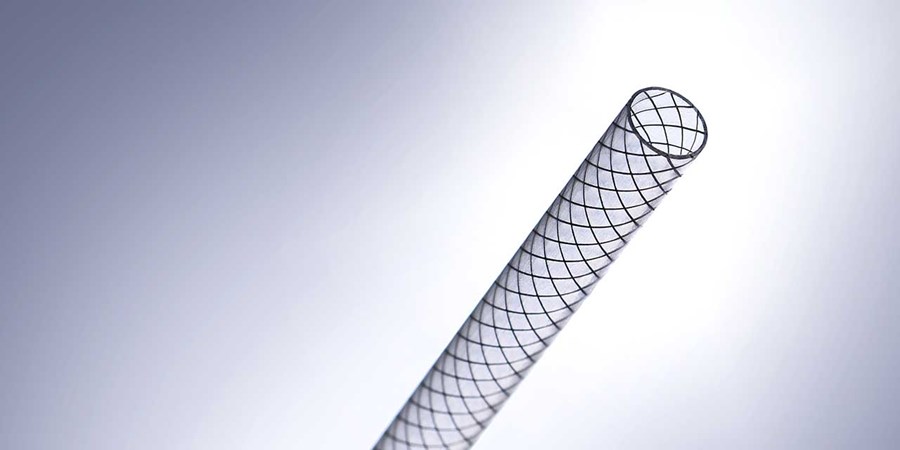
We manufacture braid- and coil-reinforced composite shafts with tight tolerances, built for high performance.

We’re the market leader in dilator and sheath technologies that support interventional therapies. Our custom vascular access components come in a wide range of sizes, polymers, colors, and value-added operations.
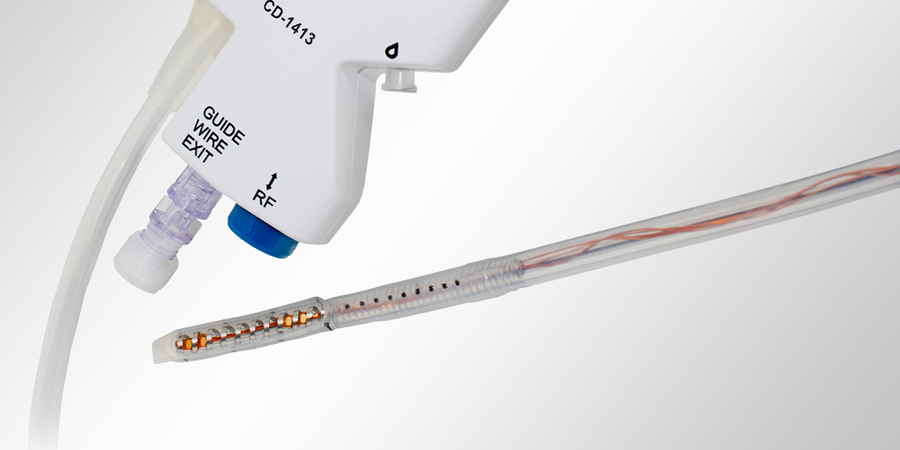
Our technical team draws on our vertically integrated capabilities in materials, extrusion, balloon-forming, molding, and secondary operations to deliver complex catheter-based devices.
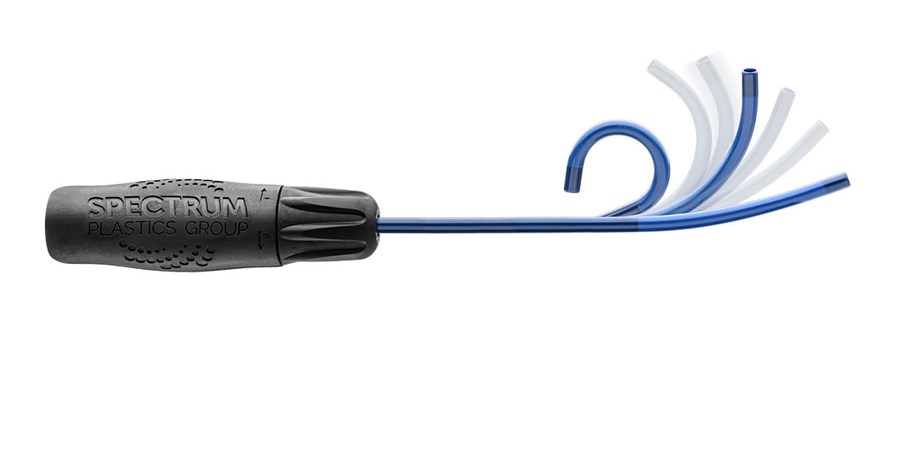
We develop and manufacture standard or customized configurations of steerable catheter delivery systems to meet your device needs. From concept to manufacturing, we offer full-service steerable catheter production.
As experts in silicone, we offer a complete catheter solution including silicone extrusion, balloon forming, shaft/funnel overmolding, manufacturing, and assembly––all backed by decades of silicone catheter innovation.
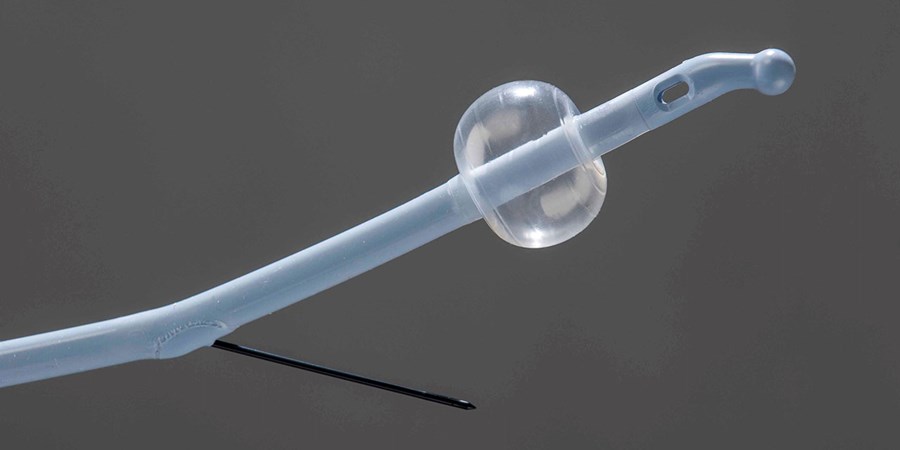
Our balloon catheters are a winning combination of our Apollo® Balloon Tubing with Earnan® Balloon Technology, globally recognized for innovation and complex technical solutions.
As medical catheters become smaller and more complex, lasers are increasingly the preferred technology for creating complex, micron-sized features in a range of materials.

DuPont™ Spectrum™ has developed groundbreaking additive manufacturing technologies, utilizing proprietary processes and medical-grade materials to create components with complex tolerances, geometries, and features.
Catheter technology has evolved significantly with innovations in material science, manufacturing precision, and component miniaturization. Advanced polymers and composite structures enable smaller profiles with enhanced torque transmission and flexibility transitions. Microfabrication techniques have improved tip designs for reduced vascular trauma, while integrated sensing capabilities provide real-time physiological feedback. These advancements collectively reduce procedure times, decrease complication rates, and enable treatment of increasingly complex anatomical conditions.
Material selection for catheters requires evaluation of mechanical performance requirements, biocompatibility profile, and sterilization compatibility. Considerations include torqueability needs, kink resistance, column strength, and flexibility gradients along the device length. Chemical resistance to medicants or bodily fluids impacts material longevity, while tissue interaction characteristics influence procedural outcomes. Application-specific factors such as radiopacity requirements and friction coefficients must also be evaluated for optimal catheter performance.
Multi-lumen catheter designs enable simultaneous functionality through segregated channels within a single device profile. This configuration allows for concurrent aspiration and infusion, pressure monitoring during interventional procedures, or guidewire passage alongside therapeutic delivery. The strategic positioning of lumens optimizes flow dynamics and mechanical performance, while maintaining the smallest possible overall diameter. Advanced manufacturing techniques ensure precise lumen geometry and consistent wall thickness throughout complex designs.
Quality in catheter manufacturing depends on rigorous process controls, validated testing methods, and comprehensive design verification. Critical parameters include dimensional consistency throughout the catheter length, bond integrity between components, and maintenance of material properties through all processing steps. Performance testing must evaluate both static and dynamic characteristics under simulated use conditions, including fatigue resistance during repeated flexure cycles. Sterilization validation ensures maintained functionality after terminal processing required for medical applications.
We’re happy to help with your projects in any way we can. Contact us and we’ll email you back the information you’re looking for, or we’ll schedule a call to discuss with you in more detail.

Spectrum™ is the starting point for medical device development requiring critical catheter components. Our off-the-shelf tubing and components are available in our Component Webstore to accelerate the design and development of your next project.

